Top marks for Mindpeak's latest lung cancer diagnosis software

Mindpeak’s PD-L1 Lung AI achieves 92% concordance in clinical DRT test without human intervention.
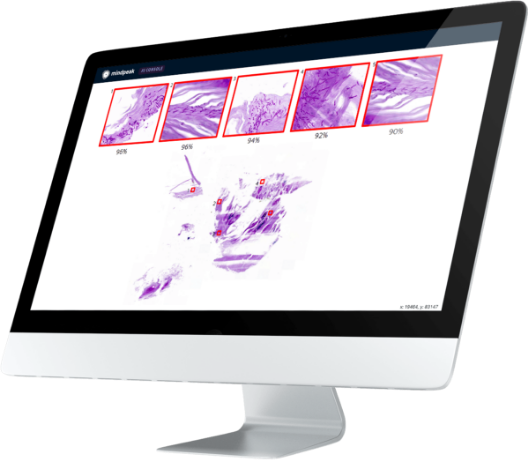
Mindpeak's AI software for scoring PD-L1 in non-small cell lung cancer (NSCLC) has passed the Digital Readout Test (DRT) of German Quality Assurance Initiative in Pathology (QuIP), the leading service provider for quality assurance in pathology in Germany. QuIP supports pathologists, pharmaceutical companies and diagnostic providers in validating and optimizing their diagnostic assessment performance. The test was performed by a fully autonomous AI, without human intervention. The AI analyzed whole slide images of NSCLC. The resulting tumor proportion score (TPS) according to the AI was submitted to QuIP. With a concordance level of over 92% with a ground truth provided by QuIP, the AI algorithm is on par with the best German labs. The “Lung (NSCLC) PD-L1 (SP263)” algorithm is CE-marked for clinical diagnostic use in NSCLC. "I am very pleased that our AI software was able to solve the DRT without human intervention on this challenging data set”, explains Mindpeak's founder and CEO Felix Faber.
Digital Readout Tests (DRTs) are a complement to the proficiency tests of QuIP. Diagnostic tissue samples are provided for assessment to participants centrally in digitized form. Participants assess samples with regard to the given criteria, such as biomarker-dependent scores or entity-related categories. After successful completion of a DRT, participants receive a certificate from QuIP. Mindpeak has been one of the first AI software developers to participate in the newly established Digital Readout Tests, pioneering this new digital validation method. QuIP has announced that in the future it will increasingly allow AI developers to participate directly with their evaluation algorithms.
“Lung (NSCLC) PD-L1 (SP263)” is one of already eleven products by Mindpeak with a CE mark to support pathologists in cancer diagnosis that the Hamburg-based company has developed and brought to market. Founded by Faber and Dr Lang, the company has been developing image recognition software for pathologists using artificial intelligence since 2018. Mindpeak has developed the first AI solution for pathology that has made it into clinical routine in both, Europe as well as the US. In partnership with international laboratories and leading pathology workflow platform providers, Mindpeak is continuously expanding its product range and developing it into an indispensable part of the digitized pathology workflow. This makes Mindpeak’s AI software the most widely integrated one worldwide.
You may also be interested in
Find out more about our AI-based diagnostics



.webp)

